The LINC 4 study demonstrated superiority of Isturisa® (osilodrostat) over placebo in achieving cortisol normalisation during the 12-week, double-blind, randomised phase (77% vs 8%, P<0.0001).
Isturisa provided rapid and sustained control of cortisol secretion in the majority of patients throughout the 48-week core phase of the study.
PUTEAUX, France, March 29, 2022--(BUSINESS WIRE)--Recordati Rare Diseases announce today the publication of positive results from the Phase III LINC 4 study of Isturisa in The Journal of Clinical Endocrinology & Metabolism.1 These data reinforce Isturisa as an effective and well-tolerated oral therapy for patients with Cushing’s disease. Isturisa is indicated in the EU for the treatment of adult patients with endogenous Cushing’s syndrome,2 a rare and debilitating condition of hypercortisolism that is most commonly caused by a pituitary adenoma (Cushing’s disease).3
The LINC 4 study augments the efficacy and safety data for Isturisa in patients with Cushing’s disease, confirming the results from the Phase III LINC 3 study. This study in 73 adults is the first Phase III study of a medical treatment in patients with Cushing’s disease to include an upfront, randomised, double-blind, placebo-controlled period during which 48 patients received Isturisa and 25 received placebo for the first 12 weeks, followed by an open-label period during which all patients received Isturisa until week 48; thereafter, patients could enter an optional extension phase.
Key findings published in the manuscript entitled ‘Randomised trial of osilodrostat for the treatment of Cushing’s disease’ include:1
-
LINC 4 met the primary endpoint: Isturisa was significantly superior to placebo at normalising mUFC at the end of a 12-week randomised, double-blind period (77% vs 8%; P<0.0001).
-
Effects of Isturisa were rapid. Over one-quarter of patients randomised to Isturisa achieved normal mUFC as early week 2 and 58% achieved control by week 5.
-
The key secondary endpoint was also met, with 81% of all patients in the study having normal mUFC at week 36.
-
Improvements in cardiovascular and metabolic parameters of Cushing’s disease, including blood pressure and blood glucose metabolism, were seen by week 12 and were maintained throughout the study.
-
Physical features of hypercortisolism improved during Isturisa treatment, including fat pads, facial rubor, striae, and muscle wasting. Improvements were observed by week 12, with continued improvement throughout the study to week 48.
-
Patient-reported QoL scores (CushingQoL and Beck Depression Inventory) also improved during Isturisa treatment.
-
Isturisa was well tolerated in the majority of patients, with no unexpected adverse events (AEs). The most common AEs overall were decreased appetite, arthralgia, fatigue and nausea.
"These results show convincingly that osilodrostat is an effective treatment for Cushing’s disease," said Peter J. Snyder MD, Professor of Medicine at the University of Pennsylvania. "Osilodrostat rapidly lowered cortisol excretion to normal in most patients with Cushing’s disease and maintained normal levels throughout the core phase of the study. Importantly, this normalisation was accompanied by improvements in cardiovascular and metabolic parameters, which increase morbidity and mortality in Cushing’s disease."
"These compelling data build on the positive Phase III LINC 3 study, published in The Lancet Diabetes & Endocrinology in 2020,4 demonstrating that Isturisa enables most patients with Cushing’s disease to gain rapid control of their cortisol levels, which in turn provides relief from a host of undesirable symptoms," said Alberto Pedroncelli, Clinical Development & Medical Affairs Lead, Global Endocrinology, Recordati AG. "Recordati Rare Diseases is committed to improving the lives of patients with this rare, debilitating and life-threatening condition. I would like to thank everyone who has contributed to LINC 4 and the LINC clinical programme."
"I had Cushing's disease for 8 years without being diagnosed," said Thérèse Fournier from L'association "Surrénales". "I was trapped in a vicious circle of missed diagnoses and worsening physical and psychological symptoms that became life-threatening. I lost everything – my job, my house, my partner, my friends – I was isolated. When I finally received my diagnosis, I was relieved because I knew the truth. Since my surgery, I have been learning to live again, enjoying the moments that make a life. I am still on the path to remission, but I feel deeply happy, even if I carry this journey that nobody can understand."
About Cushing’s syndrome
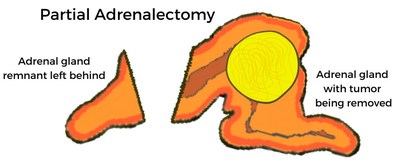
Cushing’s syndrome is a rare disorder caused by chronic exposure to excess levels of cortisol from either an exogenous (eg medication) or an endogenous source.5 Cushing’s disease is the most common cause of endogenous Cushing’s syndrome and arises as a result of excess secretion of adrenocorticotropic hormone from a pituitary adenoma, a tumour of the pituitary gland.5,6 There is often a delay in diagnosing Cushing’s syndrome, which consequently leads to a delay in treating patients.7 Patients who are exposed to excess levels of cortisol for a prolonged period have increased comorbidities associated with the cardiovascular and metabolic systems, which consequently reduce QoL and increase the risk of mortality.3,6 To alleviate the clinical signs associated with excess cortisol exposure, the primary treatment goal in Cushing’s syndrome is to reduce cortisol levels to normal.8
About LINC 4
LINC 4 is a multicentre, randomised, double-blind, 48-week study with an initial 12-week placebo-controlled period to evaluate the safety and efficacy of Isturisa® in patients with Cushing’s disease. The LINC 4 study enrolled patients with persistent or recurrent Cushing’s disease or those with de novo disease who were ineligible for surgery; 73 randomised patients were treated with Isturisa® (n=48) or placebo (n=25).1 The primary endpoint of the study is the proportion of randomised patients with a complete response (mUFC ≤ULN) at the end of the placebo-controlled period (week 12). The key secondary endpoint is the proportion of patients with an mUFC ≤ULN at week 36.1,9
About Isturisa®
Isturisa® is an oral inhibitor of 11β-hydroxylase (CYP11B1), which catalyses the final step of cortisol synthesis in the adrenal glands.2 Isturisa® is available as 1 mg, 5 mg and 10 mg film-coated tablets.2 Isturisa® is approved for the treatment of adult patients with endogenous Cushing’s syndrome in the EU and is now available in France, Germany, Greece and Austria.2
Isturisa® was granted marketing authorisation by the European Commission on 9 January 2020. For detailed recommendations on the appropriate use of this product, please consult the summary of product characteristics.2
References
1. Gadelha M, Bex M, Feelders RA et al. Randomised trial of osilodrostat for the treatment of Cushing's disease. J Clin Endocrinol Metab 2022; dgac178, https://doi.org/10.1210/clinem/dgac178.
2. Isturisa® summary of product characteristics. May 2020.
3. Ferriere A, Tabarin A. Cushing's syndrome: Treatment and new therapeutic approaches. Best Pract Res Clin Endocrinol Metab 2020;34:101381.
4. Pivonello R, Fleseriu M, Newell-Price J et al. Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol 2020;8:748-61.
5. Lacroix A, Feelders RA, Stratakis CA et al. Cushing's syndrome. Lancet 2015;386:913-27.
6. Pivonello R, Isidori AM, De Martino MC et al. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol 2016;4:611-29.
7. Rubinstein G, Osswald A, Hoster E et al. Time to diagnosis in Cushing's syndrome: A meta-analysis based on 5367 patients. J Clin Endocrinol Metab 2020;105:dgz136.
8. Nieman LK, Biller BM, Findling JW et al. Treatment of Cushing's syndrome: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:2807-31.
9. ClinicalTrials.gov. NCT02697734; available at https://clinicaltrials.gov/ct2/show/NCT02697734 (accessed March 2021).
Recordati Rare Diseases, the company’s EMEA headquarters are located in Puteaux, France, with global headquarter offices in Milan, Italy.
For a full list of products, please click here: www.recordatirarediseases.com/products.
Recordati, established in 1926, is an international pharmaceutical group, listed on the Italian Stock Exchange (Reuters RECI.MI, Bloomberg REC IM, ISIN IT 0003828271), with a total staff of more than 4,300, dedicated to the research, development, manufacturing and marketing of pharmaceuticals. Headquartered in Milan, Italy, Recordati has operations in Europe, Russia and the other C.I.S. countries, Ukraine, Turkey, North Africa, the United States of America, Canada, Mexico, some South American countries, Japan and Australia. An efficient field force of medical representatives promotes a wide range of innovative pharmaceuticals, both proprietary and under license, in several therapeutic areas including a specialized business dedicated to treatments for rare diseases. Recordati is a partner of choice for new product licenses for its territories. Recordati is committed to the research and development of new specialties with a focus on treatments for rare diseases. Consolidated revenue for 2021 was € 1,580.1 million, operating income was € 490.2 million and net income was € 386.0 million.
For further information:
Recordati website: www.recordatirarediseases.com
This document contains forward-looking statements relating to future events and future operating, economic and financial results of the Recordati group. By their nature, forward-looking statements involve risk and uncertainty because they depend on the occurrence of future events and circumstances. Actual results may therefore differ materially from those forecast as a result of a variety of reasons, most of which are beyond the Recordati group’s control. The information on the pharmaceutical specialties and other products of the Recordati group contained in this document is intended solely as information on the Recordati group’s activities and therefore, as such, it is not intended as medical scientific indication or recommendation, nor as advertising.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220325005169/en/
Contacts
Celine Plisson, MD
Medical Affairs Director
Telephone: +33(0)147739463
Email: PLISSON.C@recordati.com












































Day 16, Cushing’s Awareness Challenge 2022
in Cushing's Awareness Day, April 8
Posted
I used to carry a print out of this everywhere I go because I find it very soothing. This print out was in a plastic page saver. On the other side there is a Psalm 116, part of the post from Day Nineteen of the 2015 Cushing’s Challenge. These days, both these readings are available on my phone.
I first read this in Chicken Soup for the Surviving Soul and is posted several places online.
The Best Day Of My Life
by Gregory M Lousignont
Today, when I awoke, I suddenly realized that this is the best day of my life, ever! There were times when I wondered if I would make it to today; but I did! And because I did I’m going to celebrate!
Today, I’m going to celebrate what an unbelievable life I have had so far: the accomplishments, the many blessings, and, yes, even the hardships because they have served to make me stronger.
I will go through this day with my head held high, and a happy heart. I will marvel at God’s seemingly simple gifts: the morning dew, the sun, the clouds, the trees, the flowers, the birds. Today, none of these miraculous creations will escape my notice.
Today, I will share my excitement for life with other people. I’ll make someone smile. I’ll go out of my way to perform an unexpected act of kindness for someone I don’t even know.
Today, I’ll give a sincere compliment to someone who seems down. I’ll tell a child how special he is, and I’ll tell someone I love just how deeply I care for her and how much she means to me.
Today is the day I quit worrying about what I don’t have and start being grateful for all the wonderful things God has already given me.
I’ll remember that to worry is just a waste of time because my faith in God and his Divine Plan ensures everything will be just fine.
And tonight, before I go to bed, I’ll go outside and raise my eyes to the heavens. I will stand in awe at the beauty of the stars and the moon, and I will praise God for these magnificent treasures.
As the day ends and I lay my head down on my pillow, I will thank the Almighty for the best day of my life. And I will sleep the sleep of a contented child, excited with expectation because know tomorrow is going to be the best day of my life, ever!
When I’m feeling down, depressed or low, reading my 2 special pages can help me more than anything else.