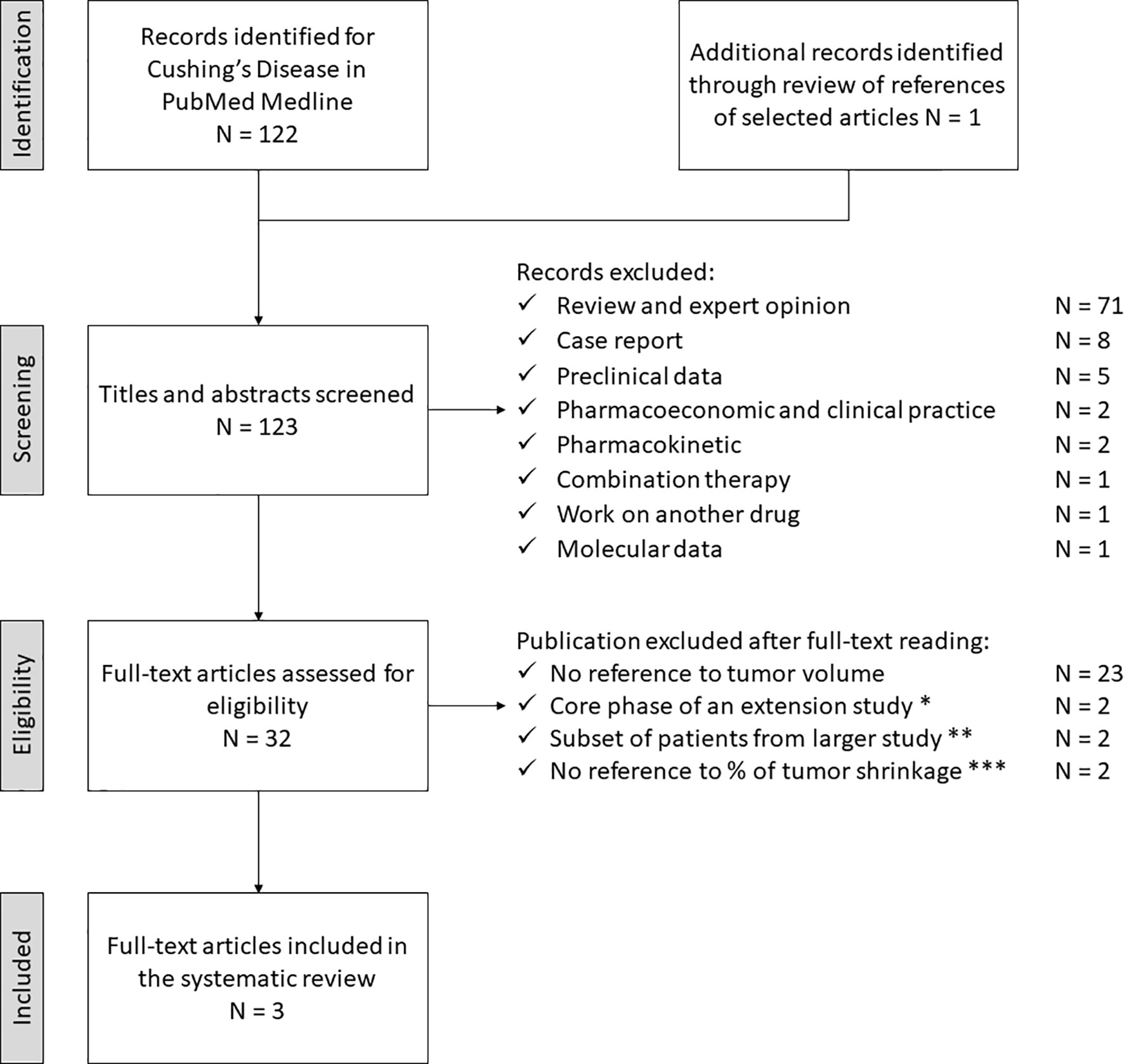
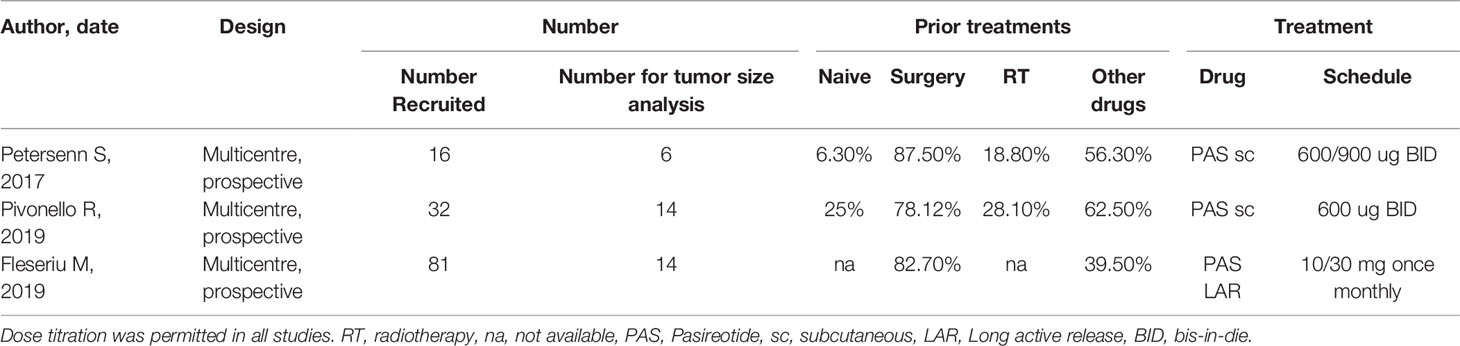
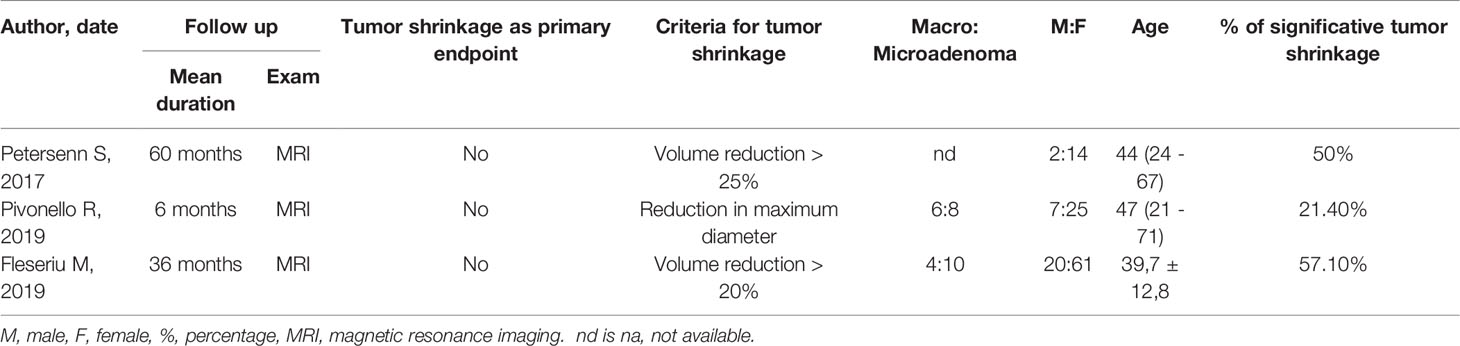
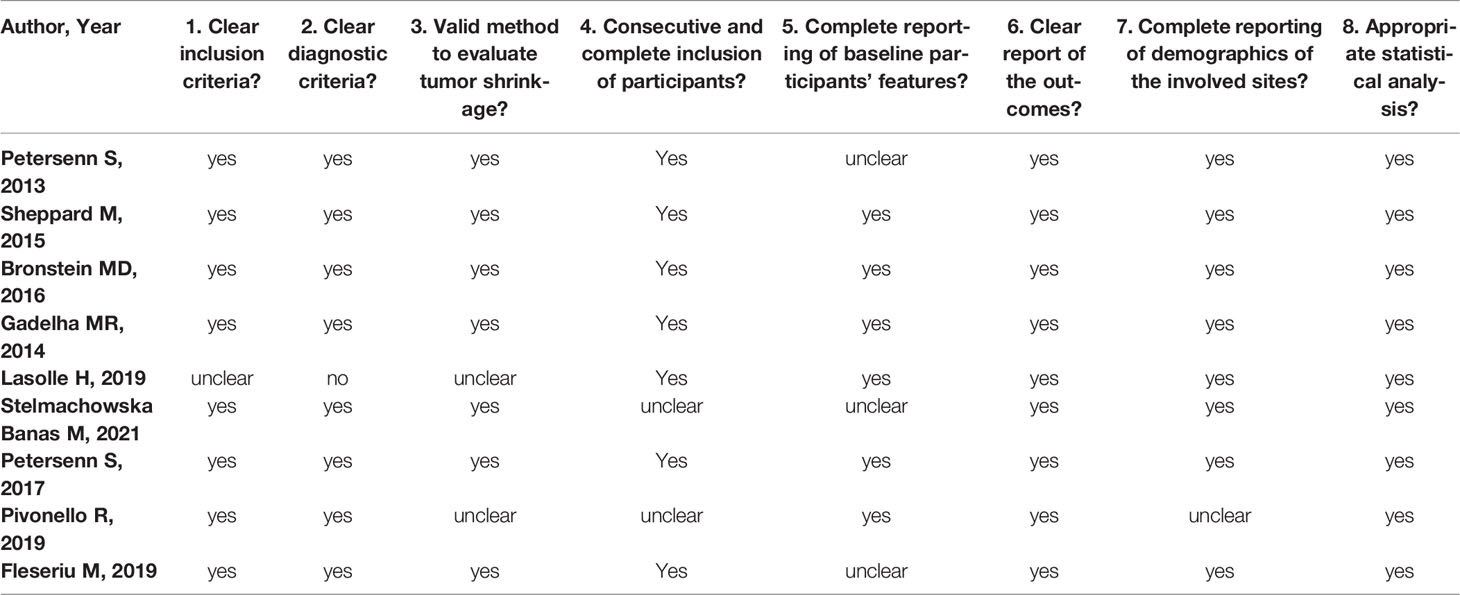
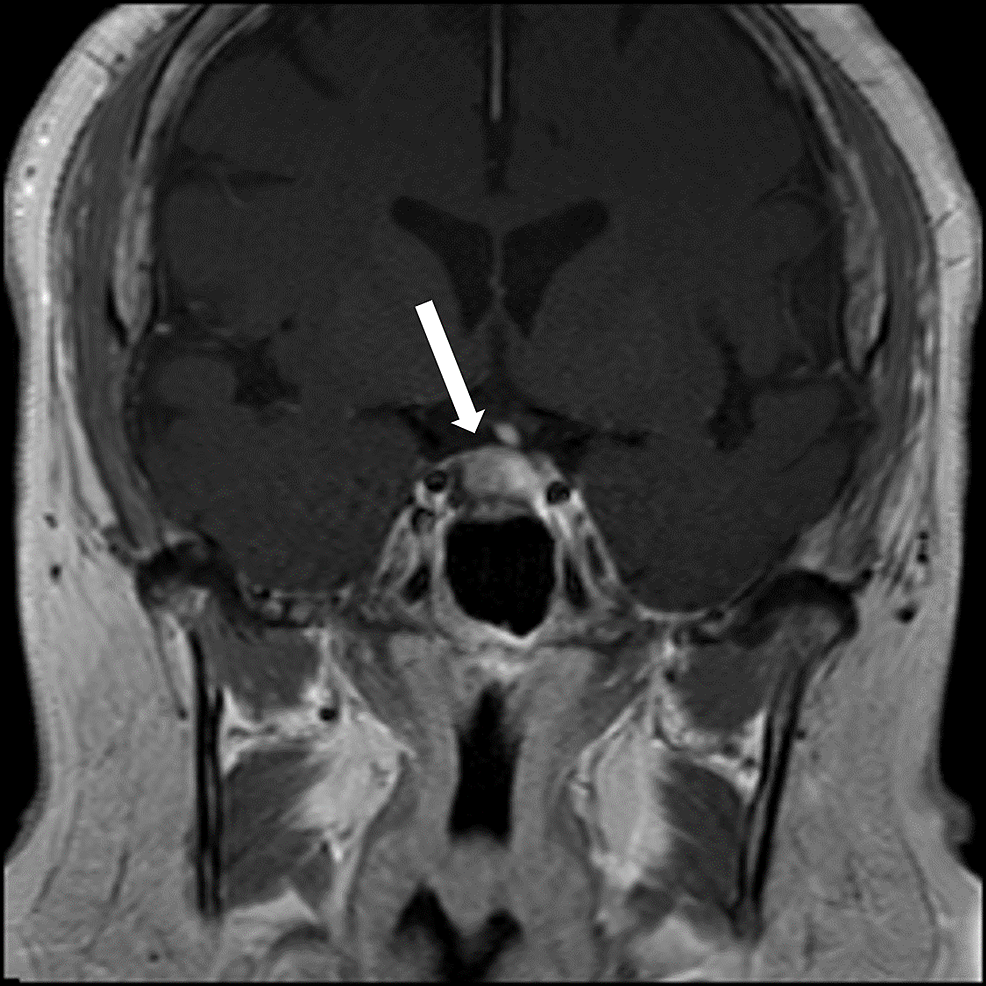
PORTLAND, Ore.--(BUSINESS WIRE)--Sparrow Pharmaceuticals, an emerging, clinical-stage biopharmaceutical company developing novel, targeted therapies for disorders of glucocorticoid excess, today presented new pharmacological data during a poster session and a Rapid Communications session titled, “HPA axis modulation by a potent inhibitor indicates 11β-hydroxysteroid dehydrogenase type 1 (HSD-1) is a main source of cortisol that can bind intracellular receptors” at the 24th European Congress of Endocrinology (ECE 2022). Sparrow scientists examined the steroid hormone changes after administration of its lead therapeutic candidate, SPI-62, an HSD-1 inhibitor, to healthy adults.
“Normalized urinary free cortisol, or UFC, is a standard therapeutic target for patients with Cushing’s syndrome,” said David A. Katz, Ph.D., CSO at Sparrow Pharmaceuticals, “But that biomarker doesn’t measure the cortisol that can access intracellular receptors and cause symptoms. UFC normalization has been shown not to correlate with clinical endpoints in patients with Cushing’s syndrome. Many patients with autonomous cortisol secretion have normal UFC, yet substantial cortisol morbidity. As we conduct clinical trials for patients with those diseases, we’re in search of better ways to measure the cortisol that makes patients ill.”
The study analyzed historical clinical trial data to better characterize how SPI-62 impacts cortisol levels and the body’s homeostatic response to those changes.
Conclusions of the study include:
- Half of hepatocellular cortisol with access to intracellular receptors is generated in healthy adults by HSD-1.
- ACTH increase compensates for the effect of HSD-1 inhibition on systemic cortisol levels.
- Secondary increases of androgen levels have not been associated to date with clinical consequences.
- Large changes of the amount of cortisol that can bind intracellular receptors, and thus cause cortisol-related morbidity, can occur independently of urinary free cortisol levels.
HSD-1 converts cortisone to cortisol in tissues in which cortisol excess is associated with morbidity including liver, adipose, bone, and brain. SPI-62 is a potent HSD-1 inhibitor in clinical development for treatment of Cushing’s syndrome and autonomous cortisol secretion, and as adjunctive therapy to prednisolone in polymyalgia rheumatica. In Phase 1 clinical trials SPI-62 was generally well tolerated and associated with maximal liver and brain HSD-1 inhibition.
To register and view the abstracts, visit ECE’s website here.















![author['full_name']](https://clf1.medpagetoday.com/media/images/author/kristenMonaco_188.jpg)
Low Blood Pressure?
in Guest Questions
Posted
From my email - I know high blood pressure is quite common with Cushing’s but has anyone who has been diagnosed experienced very low blood pressure?